26
December
2014
HYDROCARBON
ENGINEERING
The multiple popcorn observations in unexpected locations
suggest the following:
n
n
Popcorn is not exclusive to butadiene plants; it has repeatedly
occurred in locations throughout light ends: deethaniser,
depropaniser and debutaniser.
n
n
Popcorn can occur in areas with <70% bulk butadiene
concentration.
n
n
Conditions exist where localised butadiene concentration in the
vapour space can be higher than the bulk butadiene
concentrations.
n
n
Conditions exist where localised residence times are longer than
bulk residence time. Examples include porous gasket material
and pressure relief values (stagnant area with low flow). These
locations are more susceptible to initiation of popcorn.
n
n
Popcorn may propagate at lower concentrations of butadiene.
Lab studies indicate that high temperature can compensate for
low butadiene concentration.
12
n
n
C
5
dienes (isoprene and piperylene) contribute to popcorn
fouling in light ends.
Methods to identify popcorn
It is misleading to rely on visual inspection alone to diagnose an
industrial sample. Popcorn may appear similar to glassy polymer, as
they are often present concurrently. The ethylene producer should
have a means to identify popcorn polymer, and perhaps more
importantly, determine the activity. Microscopy can be employed;
these methods evaluate the morphology of the foulant but do not
quantify the activity.
Optical microscopy was used to analyse several popcorn
samples. The results suggest a distinction between samples formed in
the purification section of a butadiene unit and in areas of light ends
towers with <70% bulk butadiene. Micrographs of popcorn samples
are visible in Figure 4: micrograph A is representative of popcorn
recovered from purification tower of a butadiene unit and
micrograph B is representative of popcorn recovered from a
debutaniser reboiler. The light ends samples generally have more
discreet seeds embedded in glassy polymer. Although samples
recovered from butadiene purification sections typically contain a
mixture of a popcorn and glassy polymer, the popcorn portion
contains numerous identifiable seeds. The difference in the relative
number of identifiable seeds may be a consequence of the lower
butadiene concentration during its formation.
The styrene polymerisation popcorn test (SPPT), recently
developed by Dow, is a method to evaluate the growth activity of a
sample.
15
Although the test does not consider morphology, it
provides a strong indication of cross linked butadiene polymers
which is a characteristic feature of popcorn. SPPT is an extension of
the work of Miller et. al.,
10,11,13
who found that cross linked material can
initiate fast polymer growth in a styrene environment. Miller et. al.
used styrene vapour to generate
polymer growth, whereas the method
described herein is based on popcorn
growth in liquid styrene.
12
The styrene polymerisation
popcorn test is based on the use of a
reference polybutadiene popcorn
sample produced in the lab. The growth
rate of the reference in liquid butadiene
can be determined by the following
equation:
W=W
o
exp (kt)
Where:
n
n
W = weight of the popcorn after
time t
n
n
W
o
= initial weight of popcorn
n
n
k = growth rate constant
n
n
t = time on stream
The lab generated reference
popcorn is used to compare and
quantify the activity of industrial
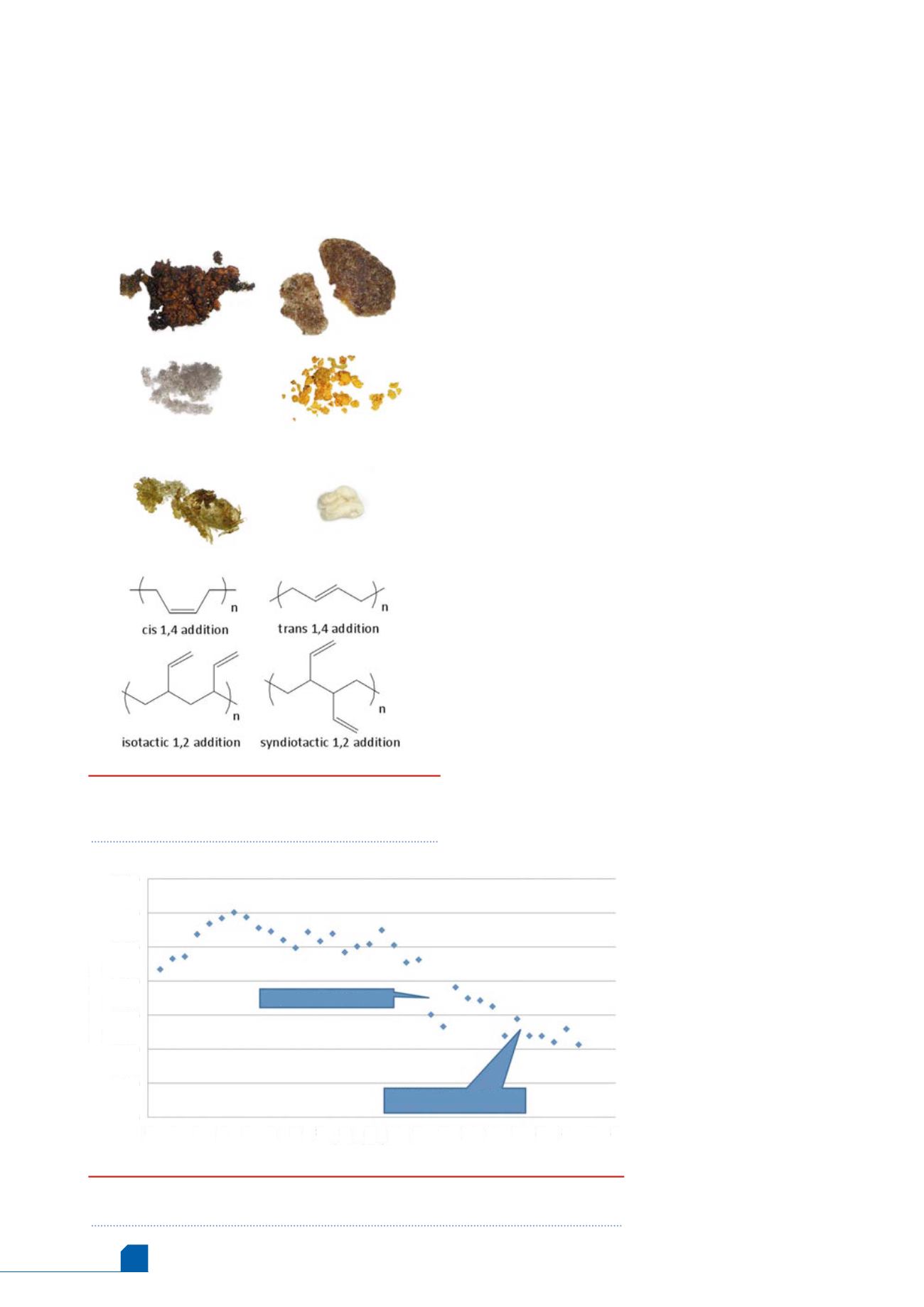
Figure 1.
Polybutadiene foulant recovered
from ethylene and butadiene plants. Chemical
structures of polybutadiene.
Figure 2.
Average popcorn growth rates as a function of the popcorn
generation.
Popcorn stored in water for 8
Generation of the popcorn
0
0.22000
0.20000
0.18000
0.16000
0.14000
0.12000
0.10000
0.08000
2 4 6 8 10 12 14 16 18 20 22 24 26 28 30 32 34 36 38
Growth rate (1/day)
Popcorn stored in water for 10 weeks


