70 |
OilfieldTechnology
February
2014
presenceof greater than1%of carbonaceousmaterials. The
amount of clays – largely kaolinite,montmorillonite,mixed layer
and illite – can vary frombeingaminor toamajor constituent of a
shale.Mullenhas shownhow themineralogical compositionof the
shale varies fromwell‑to‑well in theEagleFord shaleplay, andhis
findings are reproduced inTable1.
1
As illustrated inTable 1, shale composition can vary in
both total clay content andmineralogy. Therefore, the fluid
compositionproviding optimumperformance for drilling,
completion and stimulationoperationsmay vary across a single
field. Knowledge of these changeswithin the shaleplay is
required tomaximise the efficiency of a givenoperation type.
The shale/fluid incompatibilities causedby an improperly
formulated aqueous fluid arise from the interactionof water
with the clayminerals present in the shale. Clayminerals
are hydrous aluminiumphyllosilicates, sometimeswith
variable amounts of iron,magnesium, alkalimetals, alkaline
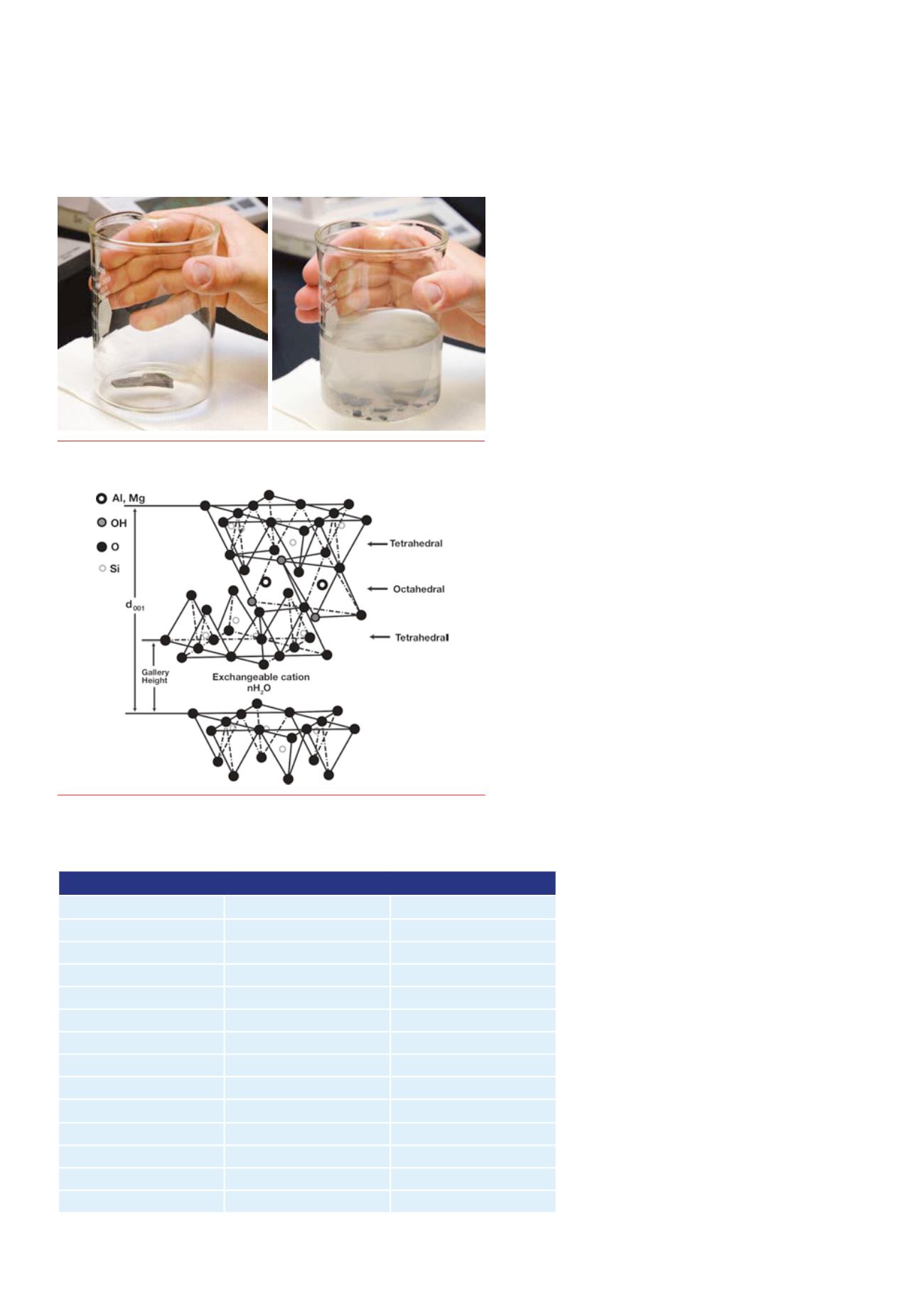
earths andother cations. Figure 2 shows the structure of
montmorillonite, also known as smectite, a common clay in
shale.
2
Montmorillonite clay forms flat hexagonal sheets or
plates, similar tomicas, which are less than twomicrons
in size. The surfaces of the clay platelets are negatively
chargedwhile the edges arepositively charged, and in an
undisturbed state thebalancing counter‑ions are usually
Na
+
. Clay particles hydrate in thepresence of water as
theNa
+
exchangeswithwatermolecules. As a result, the
montmorillonite expands considerablymore thanother
clays due towater penetrating the interlayer spaces and
concomitant adsorption. Depending on the chemical
characteristics of the shale, this can result in rapid swelling
or dispersionof the shale.
3
Indrilling, this can lead to
problems such as bit‑balling, disintegrationof cuttings,
boreholewashout, high torque anddrag and stuckpipe.
4,5
A variety of chemicals havebeenused to stabilise or
inhibitwater sensitive shales and clays over the years.
Black andHower described the use of potassium chloride
for fracturingwater‑sensitive formations in 1965.
6
This
was followedby other inorganic salts like sodium chloride
and calcium chloride,
7
aswell as tetraalkyl amine salts
8
such as ammonium chloride,
9,10
TMAC,
11
choline chloride.
12
Monomeric andpolymeric saccharides like glucosides,
13
fully
anionic PAC
14
andHEC
15
havebeenused, alongwith synthetic
polymers containing cationic,
8,16,17
anionic,
18
amphoteric
5
and
nonionic
19‑22
functionality.
As environmental toxicity has becomemore of a concern,
the use of anumber of these shale/clay inhibitors has been
discontinued inoffshore andother environmentally sensitive
areas. The toxic nature ofmany cationic
amines has limited their application in these
environmentally sensitive areas. Patel
3
compares
the toxicity of anumber ofmono‑, oligomeric‑
andpoly‑cationic amine‑based shale inhibitors
withpolyethylene imine salts
23
andoligomeric
ether amine salts
24
that show excellent shale
inhibition and are notmarine toxic.
With thewide variety of shale stabilisers
available for use, the choice of aparticular
shale inhibitormust be justifiedbasedon
several factors, such as performance, toxicity,
availability, cost and compatibility for a specific
shale formation. Shale inhibitor performance
and toxicity are the twomost important issues.
Anumber of laboratory tests exist for screening
shale reactivity todrilling and stimulation fluids.
Thesemethods include thin section analysis,
scanning electronmicroscopy (SEM), cation
exchange capacity (CEC), X‑ray diffraction
Table1. EagleFordShalemineralogical comparisonbetweenWell 1andWell 3
Mineral composition
Well 1
Well 3
Marcasite
1%
1%
Pyrite
5%
3%
Dolomite+FeDolomite
0%
2%
Calcite
55%
59%
Plagioclase
2%
3%
Quartz
15%
21%
Fluorapatite
1%
0%
Clays
20%
11%
Claycomposition
Well 1
Well 3
Kaolinite
7%
32%
Illite/smectite
53%
43%
Illite/mica
36%
25%
Chlorite
5%
0%
Figure1.
DisaggregationofMidwayshalewhenplaced intofreshwater.
Figure2.
Thestructureof2:1smectiteclays;d001referstothebasal
interlayeringspacing.ExtractedfromChenetal.(2008).Reproducedby
permissionoftheRoyalSocietyofChemistry.


